allopurinol genetic testing
In 55 1527 of patients with allopurinol-induced scar whereas only 15 181822 of the controls tested positive for the allele table 136one recent meta-analysis that consolidated all the published studies gave the odds ratios for allopurinol- induced scar in hla-b5801carriers as 73 and 165 for stud- ies using healthy controls and. Approximately 0104 of the exposed patients have been reported to develop DRESS- and SJS TEN-hypersensitivity reactions Hershfield et al 2013.

Allopurinol Metabolism Enzymes Involved Are Xdh Ao Hgprt Orotate Download Scientific Diagram
Ad Bestellen Sie den führenden DNA-Test Europas und machen Sie erstaunliche Entdeckungen.

. Gout is associated with a considerable economic burden to healthcare systems. Genetic testing to avoid allopurinol hypersensitivity Association of allopurinol SCAR and an HLA variant. Genetic Testing HLA B5801 Obtain prior to use if risk of severe Hypersensitivity skin reaction.
This means that among 100 allopurinol users with positive HLA-B5801 two patients may develop SCAR while among 100 patients tested negative almost all patients are unlikely to develop SCAR provided there are no other non-genetic factors involved. Allopurinol is commonly used in patients with hyperuricemia-associated disorders such as chronic gout uric acid nephrolithiasis and tumor-lysis syndrome via xanthine-oxidase inhibition. Gout affects around 008 of the population globally and is the most common cause of inflammatory arthritis in men1.
The indirect costs to society such as loss of productive capacity are. Therefore we determined the candidate HLA alleles for allopurinol induced DILI if the HLA allele met the following two criteria. The interactions between allopurinol its metabolite oxypurinol and T cells have been studied and evidence exists that the presence of the HLA-B5801 allele and a high concentration of oxypurinol function synergistically to increase the number of potentially immunogenic-peptide-oxypurinol-HLA-B5801 complexes on the cell surface thereby increasing the risk of T-cell.
ABCG2 is the only gene to date associated with non-response to allopurinol the most widely used ULT agent. Genetic Testing of Gout Patients for Allopurinol Sensitivity. The PPV is low at 2 and the Negative Predictive Value NPV of the genotyping test is nearly 100.
Allopurinol inhibits xanthine oxidase an enzyme that converts oxypurines to. Pre-emptive NAT2 Genetic Testing. Who and How October 10 2012 RheumatologyNetwork Staff AUDIO New ACR guidelines on gout advise considering a test for the HLA-B5801 allele linked to allopurinol sensitivity in some patients.
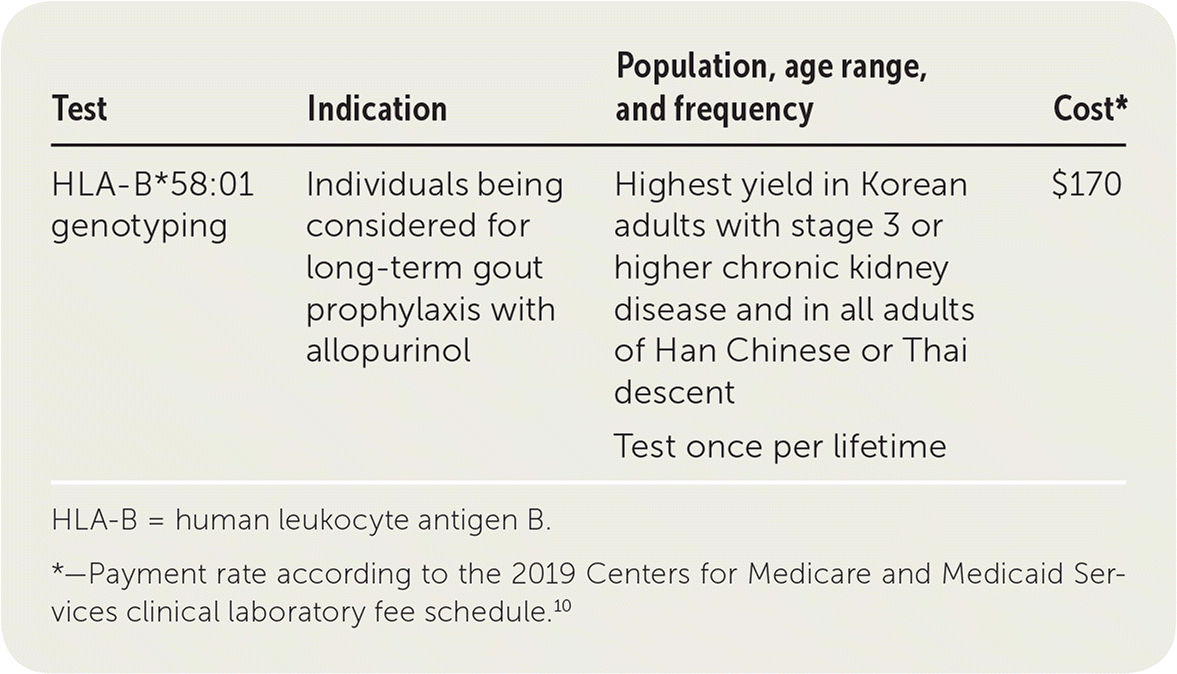
HLA-B5801 genotyping test results were available in only 14 cases. Guidelines from the Clinical Pharmacogenomics Implementation Consortium CPIC recommend HLA-B5801 genotyping be performed when considering prescribing allopurinol and that allopurinol should not be prescribed to patients who test positive for the allele due to the increased risk of SCAR2 In addition the 2020 American College of Rheumatology Guideline. Some genetic changes may make it more likely to have side effects from a medication while other genetic changes may make it less likely that the medication will help treat your symptoms.
For Allopurinol response Offered by ARUP Laboratories Molecular Genetics and Genomics. 1 the absolute difference of AF in cases and population AF is 15. When a lab uses the same methods for a test in both clinical and research settings the test appears as two separate GTR records.
Allopurinol Drug-Induced Hypersensitivity Syndrome in a Hispanic Patient. Screening for susceptibility to allopurinol-induced SCAR. This podcast by the co-chair of the panel clarifies the details.
Clinical testHelp In the US clinical tests must be performed under CLIA certification. The HSA advises that while HLA-B5801 genotyping is not routinely recommended for patients initiating allopurinol it should be considered in patients with pre-existing risk factors to identify those at greater risk of allopurinol-induced SCARs. Hans Chinese Thai Korean and African American.
Ad Bestellen Sie den führenden DNA-Test Europas und machen Sie erstaunliche Entdeckungen. The allopurinol hypersensitivity assay or HLA-B5801 test is a blood test to detect the presence of a human leukocyte antigen B HLA-B genetic variant that increases the risk of. Pharmacogenomic testing looks at changes in your genetic code called polymorphisms that can affect how you respond to certain medications.
Several studies have established a strong association between the human leukocyte antigen HLA-B5801 gene and development of Stevens-Johnson syndrome and toxic epidermal necrolysis. Risks include southeast asian esp. MyHeritage DNA - noch heute bestellen und kostenlos 1-monatiges Komplettabo erhalten.
A recent multi-national case-control study has reported allopurinol as the most common drug associated with Stevens-Johnson syndrome and toxic epidermal necrolysis. B5801 genetic testing before prescribing allopurinol to indi- viduals among populations with a high prevalence of the risk allele and. MyHeritage DNA - noch heute bestellen und kostenlos 1-monatiges Komplettabo erhalten.
In nine population-control studies HLA-B5801 was measured in 162 patients with allopurinol-induced TENSJS and 7372 patients without allopurinol-induced TENSJS. The risk of allopurinol-induced SCAR is associated with the presence. Allopurinol Drug-Induced Hypersensitivity Syndrome with Toxic Epidermal Necrolysis-Like Phenotype.
Genetic testing to avoid allopurinol hypersensitivity Bulletin for Prescribers September 2019 SHG-MKT-0196-002 Page 1 of 1 HLA testing can identify individuals at high genetic risk of developing a severe cutaneous adverse reaction to allopurinol. 2 p-value 005 from the Fisher exact test for the comparison of allopurinol cases to BioMe controls. Identification of genetic variations that predict non-response to allopurinol and uricosurics brings the possibility of genetic testing to personalise selection of ULT.
SulfamethoxazoleTrimethoprim Bactrim Drug-Induced Hypersensitivity Syndrome. Allopurinol has a long-established role in the management of hyperuricaemia and gout. Testing for the HLA-B.
Gout patients incur substantially greater direct healthcare costs compared to the healthy population23. The pooled sensitivity specificity LR LR- DOR and AUC were 078 95 CI 071-085 096 95 CI 096-097 1423 95 CI 789-2563 029 95 CI 016-054 835 95 CI 507-1374.

Allopurinol 300 Mg Obat Apa Nonflamin 34 Usd Allopurinol 300 Mg

Uric Acid Lowering Drugs Pathway Pharmacodynamics

Genetic Testing Of Gout Patients For Allopurinol Sensitivity Who And How

Allopurinol Metabolism Enzymes Involved Are Xdh Ao Hgprt Orotate Download Scientific Diagram

Allopurinol Hypersensitivity Assay Hla B 58 01 Genotyping
Allopurinol Hypersensitivity Assay Hla B 58 01 Genotyping

Use Of Hla B 58 01 Genotyping To Prevent Allopurinol Induced Severe Cutaneous Adverse Reactions In Taiwan National Prospective Cohort Study The Bmj
Frontiers A Head To Head Comparison Of Benzbromarone And Allopurinol On The Risk Of Type 2 Diabetes Mellitus In People With Asymptomatic Hyperuricemia

Allopurinol Medication Best Place

Genetic Testing In Clinical Settings American Journal Of Kidney Diseases

Allopurinol Metabolism Enzymes Involved Are Xdh Ao Hgprt Orotate Download Scientific Diagram
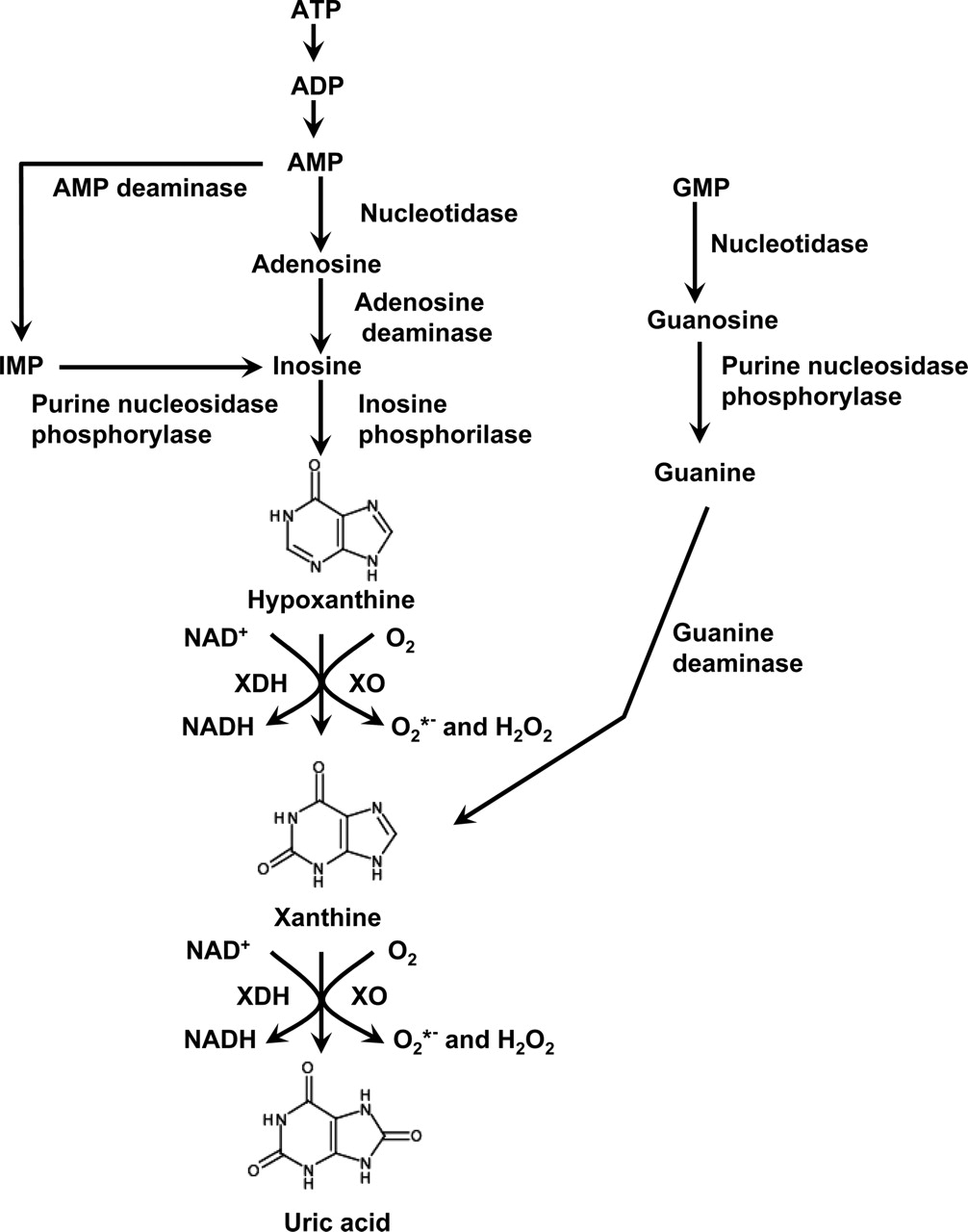
Therapeutic Effects Of Xanthine Oxidase Inhibitors Renaissance Half A Century After The Discovery Of Allopurinol Pharmacological Reviews
Treatment Options For Gout 31 03 2017
Arzneimitteluberempfindlichkeit

Allopurinol Medication Best Place

Personalizing Therapy For Older Adults With Acute Myeloid Leukemia Role Of Geriatric Assessment And Genetic Profiling Cancer Treatment Reviews
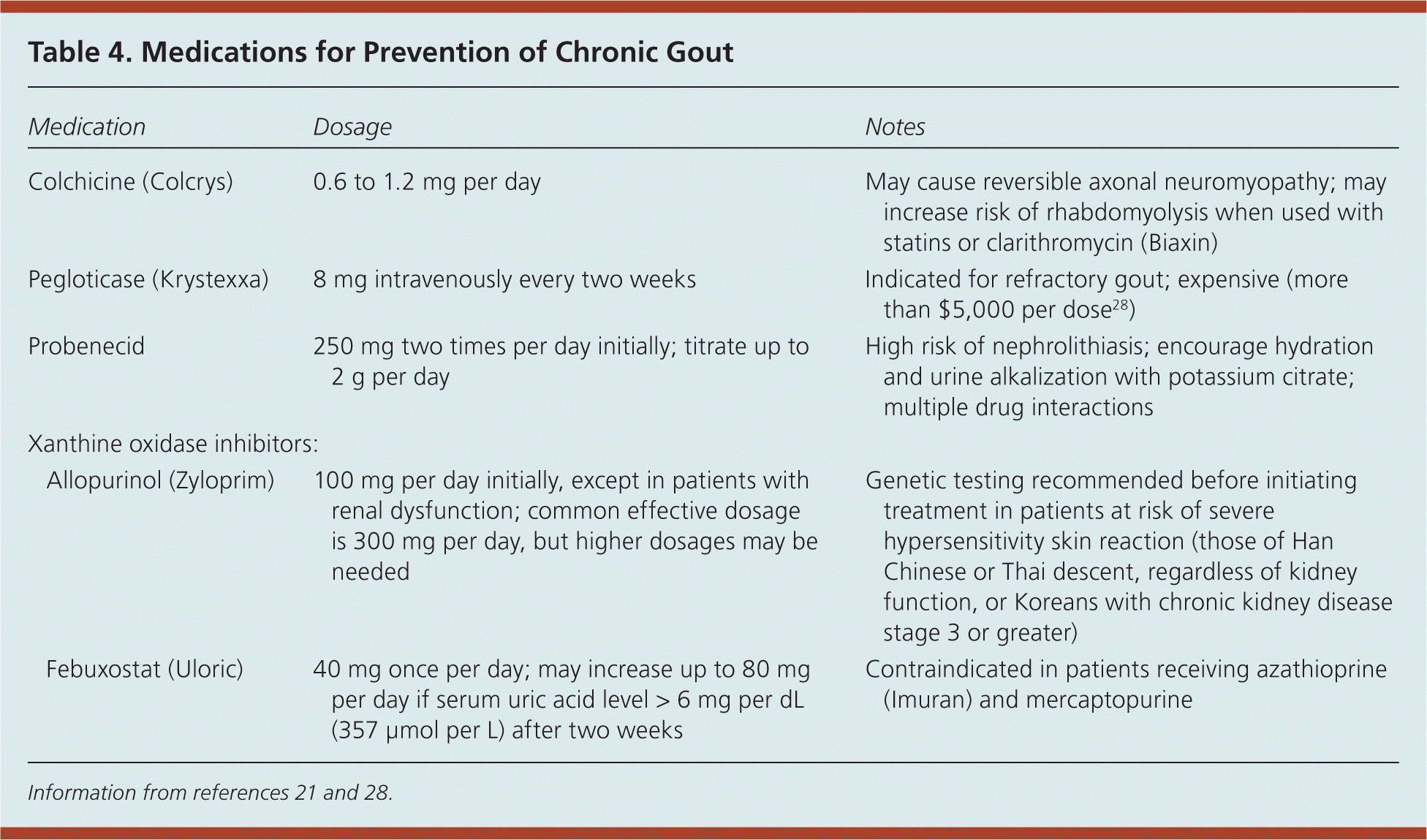
Diagnosis Treatment And Prevention Of Gout

The Reaction Cascade Of Allopurinol In Xanthine Oxidase Inhibition Download Scientific Diagram

Allopurinol Metabolism Enzymes Involved Are Xdh Ao Hgprt Orotate Download Scientific Diagram
0 Response to "allopurinol genetic testing"
Post a Comment